Platform
All features to power your data
Pharmaceutical
Listen to patients without drowning in compliance
Datashake unlocks what patients are saying online — so you can innovate products, tailor commercial strategies, and map patient journeys. Our intelligent filtering ensures you stay compliant without overwhelming your pharmacovigilance team.
Trusted by global companies



150
+
sources and counting
98.3
%
uptime SLA
5
+
years of historical data
What pharma teams build
with Datashake
Adverse event signal detection
Competitor & market intelligence
Patient journey mapping
Treatment perception & sentiment analysis
Pharmacovigilance automation
KOL & congress monitoring
App & device feedback analysis
Disease community intelligence
Adverse event signal detection
Competitor & market intelligence
Patient journey mapping
Treatment perception & sentiment analysis
Pharmacovigilance automation
KOL & congress monitoring
App & device feedback analysis
Disease community intelligence


Listen to every patient voice—without the compliance headache
Pharma companies avoid listening to patients online because of the compliance burden—every adverse event mention triggers mandatory reporting. Datashake changes the equation.
We aggregate patient discussions from 150+ sources, with intelligent filtering that lets you extract commercial insights and patient journey data without flooding your PV team with noise. Your brand teams get the patient intelligence they need. Your PV team gets only the critical signals that matter. Everyone stays compliant.
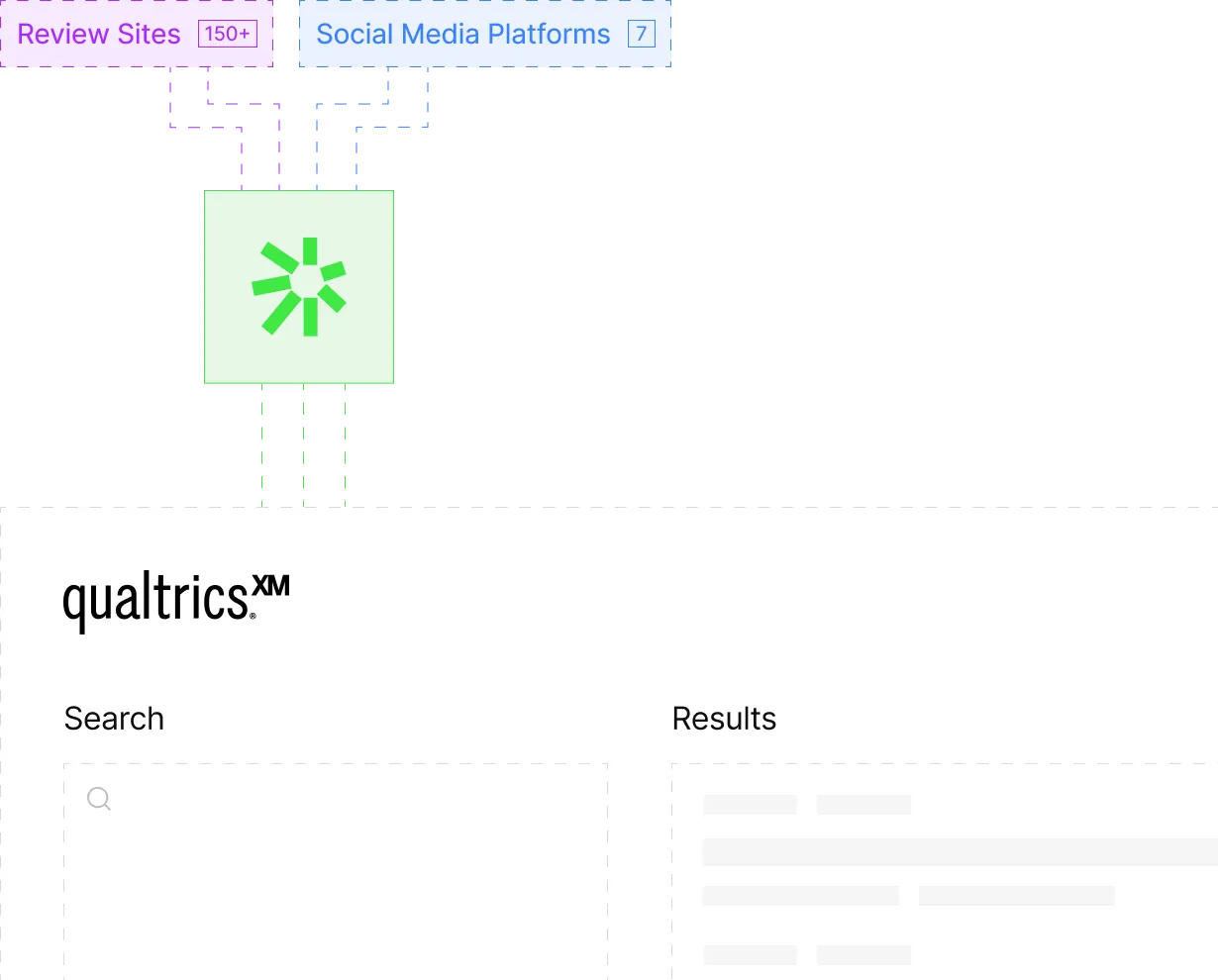
Unlock patient insights your competitors ignore
Most pharma companies deliberately don't listen to public patient discussions. You can—without the usual compliance overhead. Access the unmet needs, treatment experiences, and sentiment patterns that drive product innovation and commercial strategy.
Start filtering intelligently, not blindly
Our algorithms distinguish between a patient mentioning a headache and a genuine critical adverse event. Configure your tolerance level. Surface only what matters to your PV team—whether that's 10 reports per day or 50.
From raw data to doctor-ready content
Turn patient insights into materials that earn physician attention. Medical reps using patient-derived intelligence get meetings that sales reps can't. Move beyond white-coat science to warm, patient-first engagement.


Scale patient intelligence across brands and borders
Global coverage, one system
Multi-language, multi-region patient data collection powers multinational operations. Monitor patient discussions across any geography without deploying regional infrastructure or managing fragmented vendors.
From one brand to your entire portfolio
Add new therapeutic areas, devices, or formulations without rebuilding your infrastructure. Same workflows, same quality, whether you're monitoring one product or fifty.
Real-time or historical—your choice
Urgent signal? Get real-time alerts. Strategic planning? Query 5+ years of historical data. Baseline analysis? Track trends over time. One system, flexible delivery.
Compliance built in, not bolted on
100% pre-login public data with complete audit trails. No patient privacy exposure. PV-ready when your pharmacovigilance team needs it, commercially valuable always.



Review Management Services
I’ve been using Datashake for over six years, and it’s been a big part of our business growth during that time. It has really helped improve our operations by providing consistent, reliable data that we can easily plug into our workflows.
Tech Lead
Review Management Services
Partnering with Datashake has been a great experience. Their customer success team is responsive, knowledgeable, and always quick to help. They’re open to evolving the product as our needs grow, and their framework makes scaling up or down extremely easy.
Senior Technical Partner Manager
Review Management Services
I’ve been using Datashake for over six years, and it’s been a big part of our business growth during that time. It has really helped improve our operations by providing consistent, reliable data that we can easily plug into our workflows.
Founder & Product Lead


How Datashake powers pharmaceutical intelligence
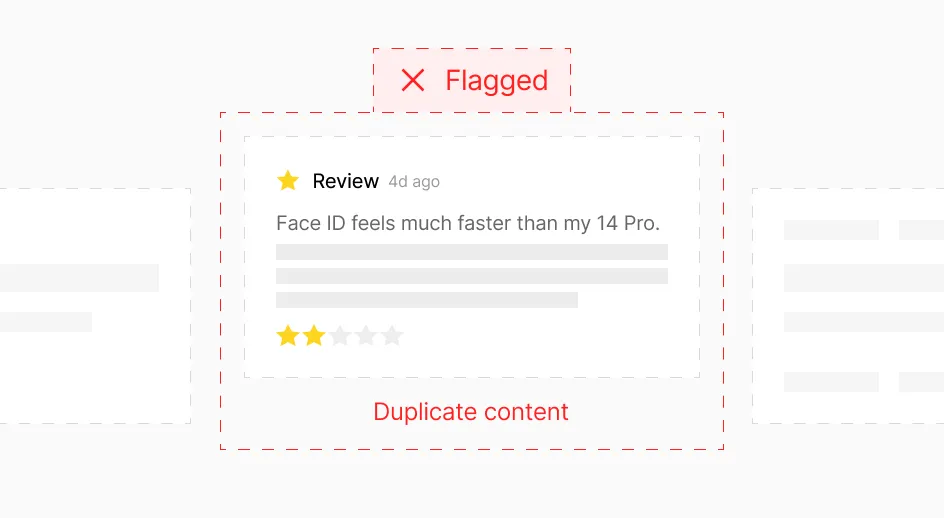
Accelerate case review with clean, contextualized signals
Eliminate noise through high deduplication accuracy. Surface complete conversation threads revealing full patient context. Leverage rich metadata to prioritize and validate signals. Integrate seamlessly with PV systems through standard JSON.
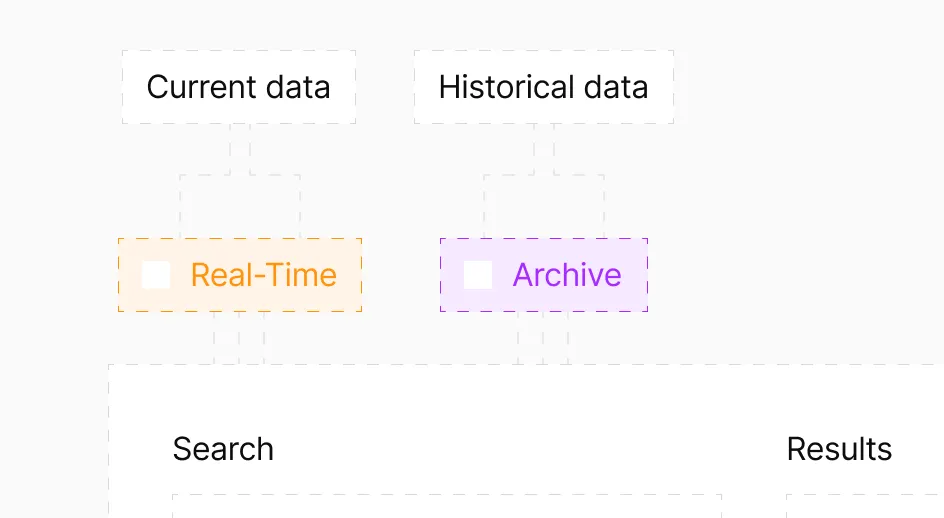
Detect emerging risks while tracking long-term patterns
Catch adverse events as they surface with real-time monitoring. Query historical data to establish baselines and identify trends. Switch between urgent surveillance and strategic analysis instantly – one system handles both.

Plug into workflows and empower teams
Connect APIs directly to case management systems and safety databases. Enable specialists and medical reviewers to explore signals independently through Datashake Hub. Execute flexible queries supporting daily screening and comprehensive investigations.




Launch comprehensive coverage in weeks
Step 01
POC with full access
Get complete API access to all 150+ sources. Test real queries with real data. Our team helps validate coverage, performance, and fit for your use cases.
Step 02
Define your solution
Configure source priorities, delivery methods, update frequencies, and pricing that supports your margins as you scale customers.
Step 03
Seamless onboarding
Dedicated technical support guides your integration. API optimization, workflow setup, and go-live validation so that you can get reliable performance from day one.


Customer Success
Put Datashake to the test for free
In a short benchmark trial, we’ll use your own search parameters to show how Datashake uncovers all the data points your current tools miss across social media, review sites, and niche sources.
Free, no-commitment benchmark test
Use your existing queries or filters
Compare results side-by-side
See how much more you could be capturing



FAQs
Your questions,
answered
We're worried about triggering more AE reports than our team can handle. How does Datashake address this?
We hear this from every pharma company we talk to. Datashake's filtering is configurable—you can set thresholds to surface only critical signals while using aggregated insights for commercial purposes. We can work within your PV team's capacity constraints.
Can you cross-reference social media mentions with official AE reports?
Yes, if you have API access to official reporting databases, we can integrate to identify net-new cases that haven't been formally reported—helping you catch emerging signals that matter while filtering out what's already known.
What sources do you cover beyond mainstream social media?
We pull from 150+ sources including pharma-specific communities, patient forums, app reviews, video platforms, and more. We cover sources that traditional social listening tools miss entirely.
We're a B2B supplier (ingredients, devices, packaging). Is Datashake relevant for us?
Absolutely. Suppliers use patient insights to help their pharma customers develop more competitive products. Understanding patient pain points—administration issues, side effects, formulation preferences—helps you sell solutions to real problems.
How is Datashake different from social listening tools?
Traditional social listening tools aren't built for pharma. They misclassify medical terms, lack coverage of key patient communities, and don't help with compliance workflows. Datashake is purpose-built for healthcare—structured data, intelligent filtering, and PV-ready outputs.
Who typically uses Datashake within a pharma company?
Brand leads and commercial teams use it for patient insights and competitive intelligence. Patient support program owners use it for journey mapping. Medical affairs uses it to generate physician engagement content. PV teams receive only the filtered, critical signals.
How long does it take to get started?
Most teams are up and running within weeks. We start with a free benchmark test using your own search parameters, then configure source priorities and filtering thresholds to match your needs before go-live.

